ISO 13485 Certification - Chicago - New York
ISO 13485 is an internationally accepted quality system standard for the medical device industry. The latest version of the standard is ISO 13485: 2016. Any organization with the scope of manufacturing, designing, or servicing medical devices can implement ISO 13485.
ISO 13485 is a mandatory requirement for CE Certification; after technical file approval, the notification body will conduct an onsite audit to assess the level of ISO 13485 implementation. The notification body will issue the CE Certificate only if the ISO 13485 implementation is satisfactory.
ISO 13485 Certification is not required per the standard, but most medical device importers and distributors ask for a third-party ISO certificate to prove proper ISO 13485 implementation.
GMP (21 CFR 820 ) is a mandatory requirement for US Medical device companies; implementing ISO 13485 will help cover more than 90% of GMP requirements. An integrated quality manual and procedure will help US companies comply with GMP and EU requirements.
ISO 13485 Consultants
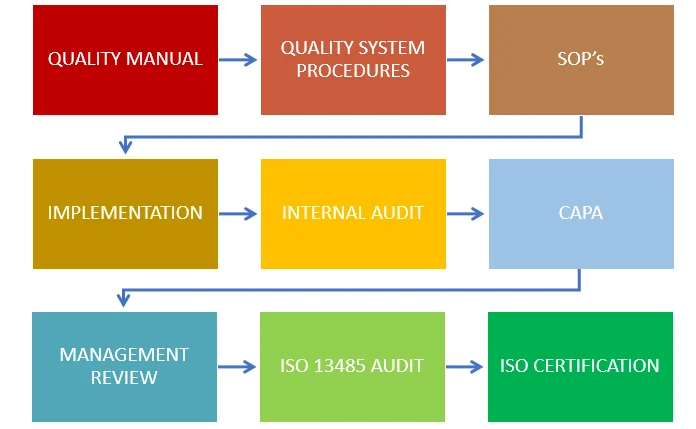
Our experienced ISO 13485 consultants will help you to prepare and implement an integrated quality system for ISO and GMP (21 CFR 820).
Our service includes.
- Prepare Quality Manual
- Prepare Quality System Procedures
- Assistance with ISO/GMP implementation
- Certification Body audit
Our Consulting Services include
For more information, Please contact us with detailed information.
LIBERTY MANAGEMENT GROUP LTD.
Chicago
75 Executive Drive, Suite 114
Aurora, IL - 60504
Phone : (630) 270-2921
Fax : (815) 986-2632
E-mail : info@libertymanagement.us
New York
100 Duffy Avenue
Hicksville, NY 11801
Phone : (516) 244-2376
E-mail : newyork@libertymanagement.us